
Metallic bonding
-
Delocalized valence electrons move freely through the
metal. -
The attraction between these electrons and the
cations holds the piece of metal intact. -
Tight packing of cations and delocalized electronstransmit kinetic energy rapidly.
-
Electrical conductivity
● The delocalization electrons enables free movement in
response to electric fields. -
Malleability
● Individual atoms are not held to any other specific
atoms, hence atoms slip easily past one another.

Ionic bonding
-
Ionic bonding is an electrostatic attraction between oppositely charged ions.
-
One ore more electrons are transferred from the outer
shell of one atom to the outer shell of another atom. -
The charge of an ion depends on the number of
electrons the atom needed to loose or gain to achieve
a full outer shell.
2 Na(s) + F2 (g) → 2 NaF (s)
01

-
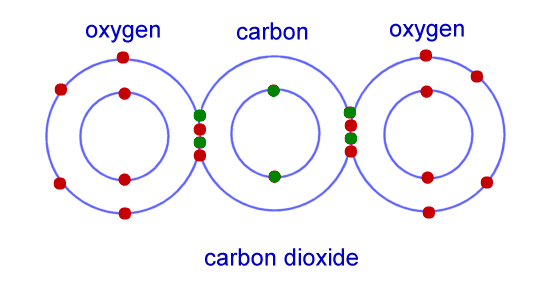
Covalent bonding is the electrostatic attraction between a pair of electrons and positively charged nuclei.
-
The more pairs of electrons there are in a covalent bond:
- the shorter the bond length
- the stronger the bond -
Multiple covalent bonds
● Single bond: One shared electron pair with one electron
from each atom.
● Double bond: Two shared electron pairs with two
electrons from each atom.
● Triple bond: Three shared electron pairs with three
electrons from each atom.
Covalent bonding

VIDEO
03
CLICK THIS TO SEE THE VIDEO
VIDEO
03
CLICK THIS TO SEE THE VIDEO
IONIC BONDING
02
EXAMPLE

COVALENT BONDING

METALLIC BONDING

